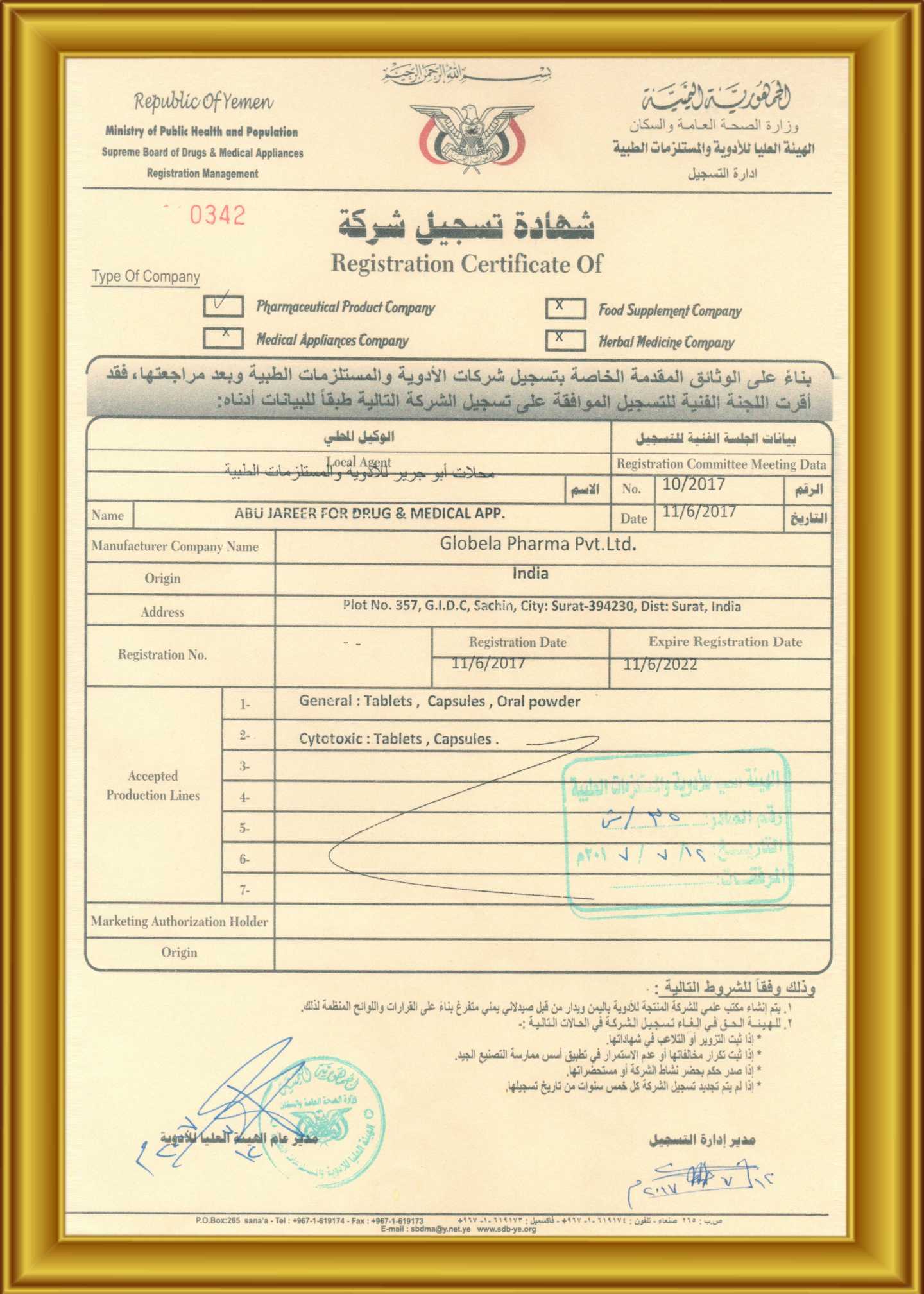
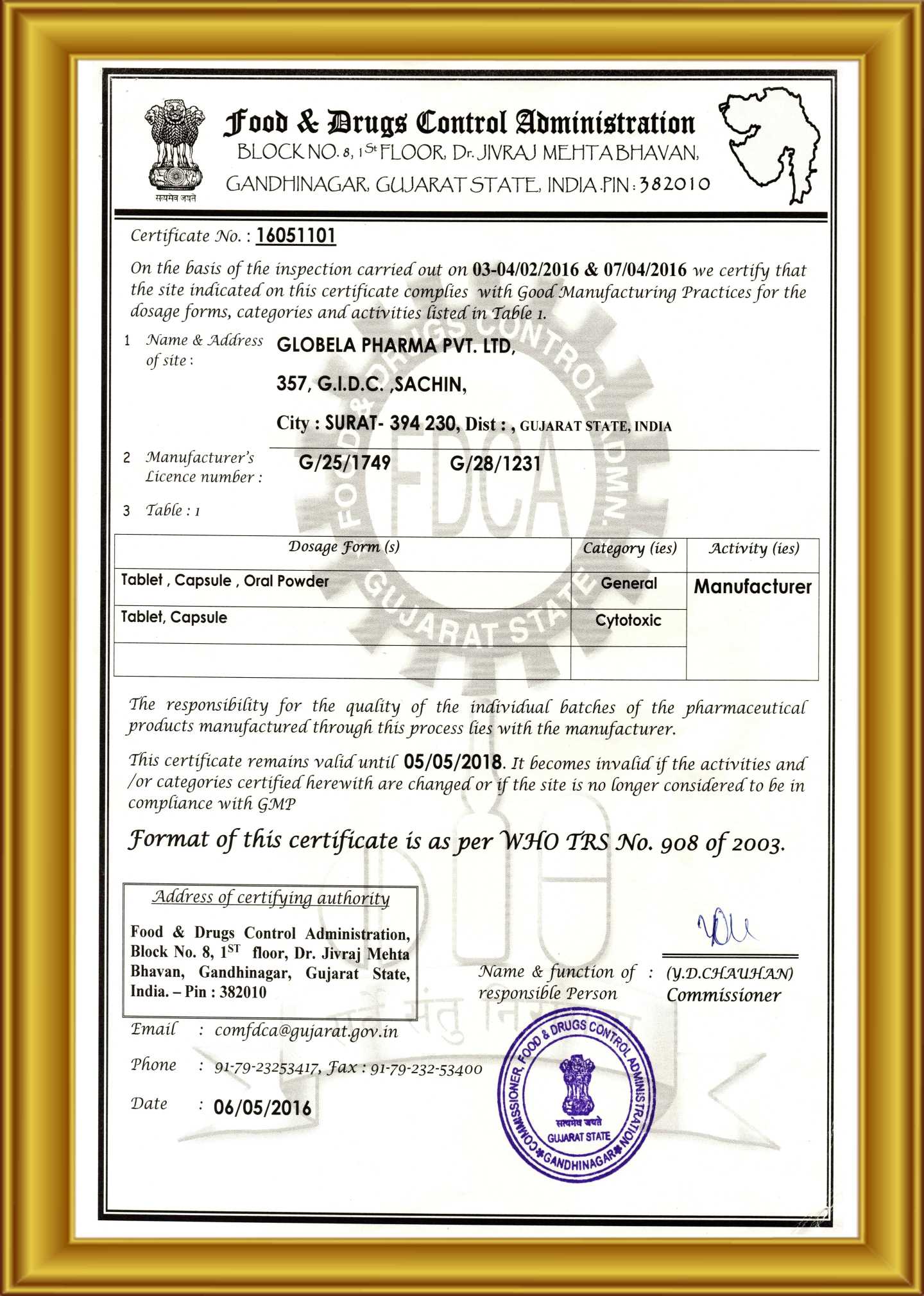
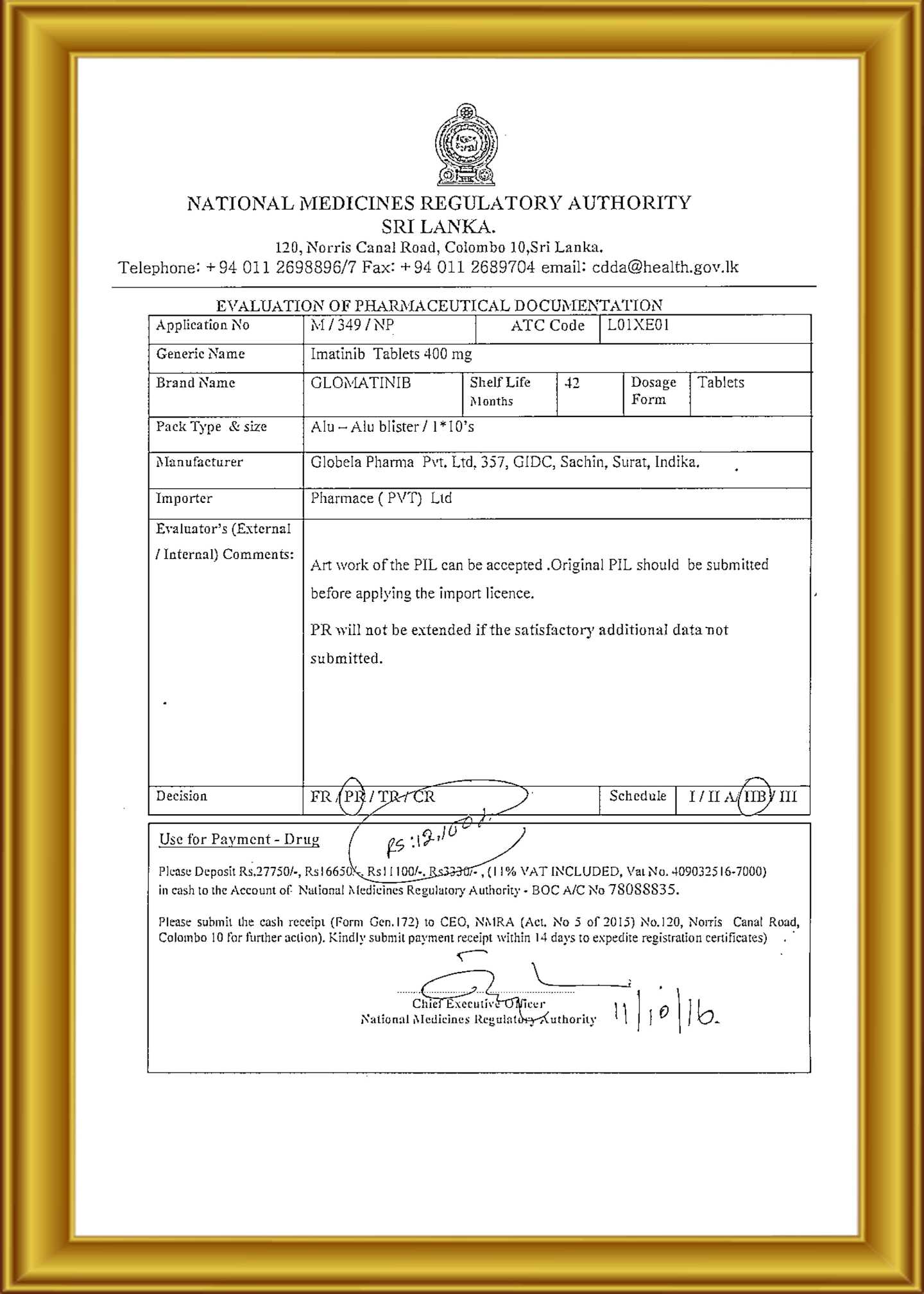
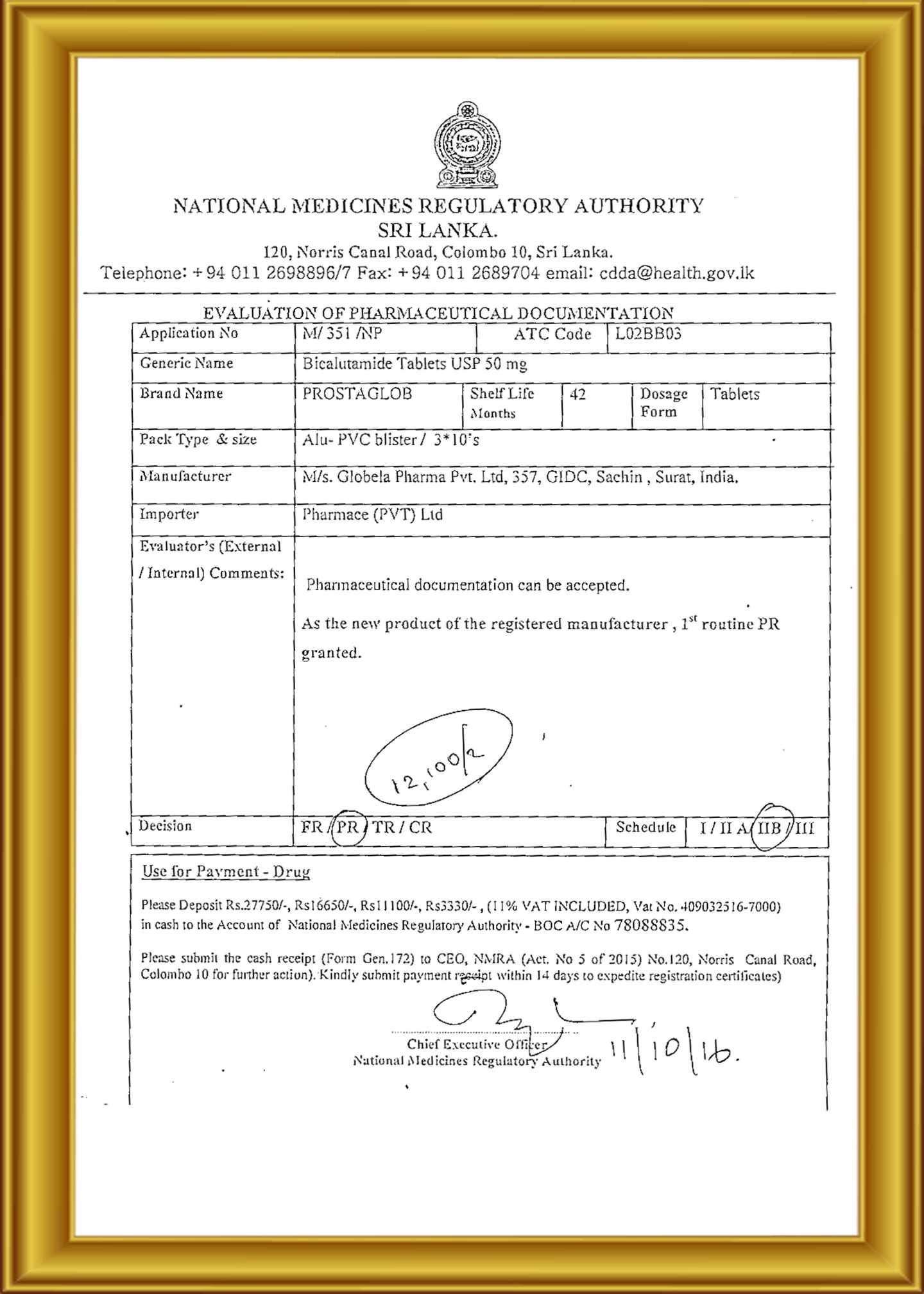
Globela Pharma Pvt ltd start in India 2006 by Highly technical experience group of people who have experience formulation, F&D , R &D , API , Intermediate more than 25 year . Company aim to establish cGMP facility for oral solid dosage ( OSD ) to cater Generic pharmaceutical product in India and export market ( ROW country ) . The Company have In-house R &D center recognizes by India Govt. ( DSIR ) for development formulation and Novel new formulation development Phase I & Phase II trials to BE clinical study . R &D facility & team are experience to handle Cytotoxic (cancer medicine) , Conventional pharmaceuticals. Globela committed for high quality pharmaceutical formulations in various forms with a team of qualified & experienced technocrats in R&D, Q.A., Q.C. & other production divisions. Globela Pharma Pvt ltd upcoming with new facility for regulatory compile EU GMP , MHRA, US FDA for OSD dedicated for regulatory market. We manufacture several intermediate chemicals, Active Pharmaceutical Ingredients & Semi-finished Dosage Forms (Pellets & Granules) and Finished Formulations. Our expertise in the field won us numerous clients in India as well as across the globe. The company was incorporated in the year 2006 under the Companies Act 1956 as a private limited company. The object of the company is to run the factory for the production of pharmaceutical items.